Brain Acoustic Monitor (BAM)
Active Signal Technologies has developed a brain acoustic monitor that has been tested in the R Adams Cowley Shock Trauma Center (STC)1 for 10 years. More recently, it has been tested for concussion detection at the Air Force Academy in parallel with neuropsychological evaluation. It was also evaluated by the DoD in the San Antonio Military Medical Center (SAMMC) for returning wounded warriors with head injuries. A new initiative currently underway at STC is looking at early detection of incipient cerebral vasospasm in severe TBI cases. BAM testing for sports concussion assessment in college and high school athletes has also been initiated by the University of Virginia Brain Injury & Sports Concussion Institute. This device, in its most fundamental principle of operation, is a brain stethoscope with a display. That is to say that although not audible by conventional or electronic stethoscopy, arterial pulse waves entering the brain produce predictable deformation of the skull that can be detected by sensors closely coupled to the skin of the forehead. And similar to conventional stethoscopy, 'normal' signals will appear as a normal arterial waveform. Conversely, distorted waveforms are associated with some anomaly in brain physiology, generally temporary as with mild traumatic brain injury. This process is discussed more thoroughly further on.
Over the course of its development, this system has evolved from a laboratory signal analyzer to a hand held PDA system (shown below), the BAM evaluates sensor inputs in both time and frequency domains, autogrades the results based on clinical evaluations of over 900 trauma patients, and displays the results in an easily readable, red / green format. The principle source of funding has been from NINDS with additional funding provided by the Army early in development and the Air Force more recently.2,3
Ruggedized PDA version of the Brain Acoustic Monitor.
In both the hospital and prehospital scenarios the BAM system has demonstrated sensitivity to TBI > 95% when compared to CT scans. In addition it has shown the capability to identify brain disturbances such as those occurring in concussion, a capability useful in both civilian triage and military fit-for-battle assessment of injured warfighters.
Modular USB version of the BAM system including the data acquisition version of the noise immune stethoscope for CCATT Critical Care Air Transport
Detection and Diagnosis of Brain Injury
At present, field diagnosis of TBI from blast or blunt force trauma is based solely on physical examination. Advanced assessment of TBI requires computed tomographic scanning (CT) or MRI, while measurement of ICP demands the placement of an invasive sensor through a hole drilled in the skull4. In contrast, the BAM5,6 has been clinically demonstrated to non-invasively detect even subtle brain injury through passive acoustic monitoring using small contact sensors and a handheld computer acquisition system. The underlying theory is that there is an acoustically detectable change where TBI occurs, with the accompanying physiological alterations corresponding to potential changes in flow noise resulting from damage to the cerebrovascular system. These result in a change in the brain’s acoustic response to its internal arterial excitation signal, as measured at the surface of the forehead and compared to that measured at a reference point, such as the radial artery. This comparison provides a baseline measurement of the person’s characteristic ‘healthy’ signal at the radial artery with which to compare the brain signal in cases where the patient is hemodynamically stable—the normal condition of most brain injured patients.
In the implementation, the BAM measures both the time variant amplitude of pulse waveforms as well as the frequency response of those waveforms. Examples are shown below to illustrate the acoustic properties of normal subjects compared with those with TBI. The first set shown in Figure 1 is from a normal patient.
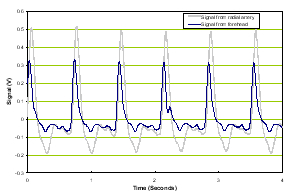
a) Time domain |
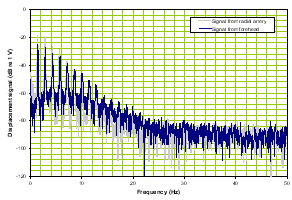
b) Frequency domain |
Figure 1. Normal time domain (left panel) and normal frequency response (right panel).
The forehead signals (blue) resemble those from the artery (gray) in general morphology. The panel on the right is of the frequency response of the two which again is similar in the overlays and the slopes of the signals. We compare these two signal sets with a patient with severe TBI as shown in Figure 2.
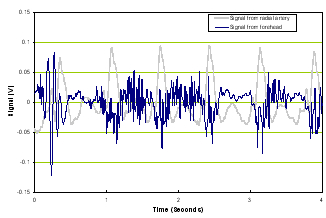
a) Time domain |
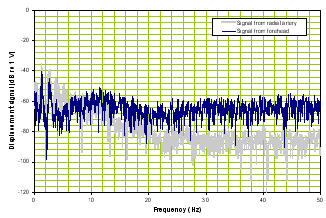
b) Frequency domain |
Figure 2. Time domain of TBI patient (left panel) and frequency response of a TBI patient (right panel).
Note that compared with Figure 1, Figure 2’s time signal from the brain is very different from the reference signal and the frequency responses of the two diverge.
With regard to signal evaluation, the BAM system has a signal processing algorithm that processes the frequency domain signals from the radial artery and the brain and sets allowable boundaries of divergence. If the patient is within those boundaries, the patient is considered ‘normal’ (BAM negative) and if outside, is considered suspicious for pathology (BAM positive). An actual example from the BAM system of a ‘normal’ relative frequency response extracted from a PDA-based BAM unit after reading a healthy volunteer is shown in Figure 3. Although we show this as an example of the raw data, the actual display in the system is a red / green display indicating the results of all sensor outputs.
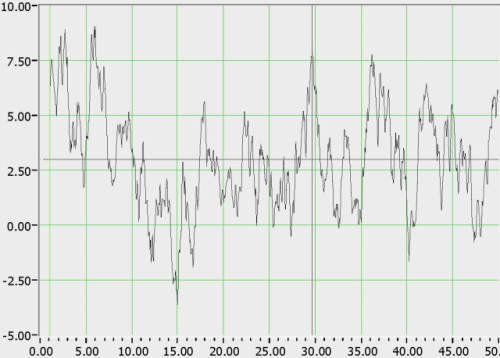
Figure 3. Relative frequency response of brain vs. artery from a healthy volunteer.
The signal is characterized by ~5 dB deviation around a mean, and >90% of ‘normals’ tested exhibited <10 dB deviation from the mean. By contrast, the relative frequency responses of persons with TBI exhibit a broad deviation (>10 dB) above the mean.
Over the course of testing, BAM has shown a disturbance in the acoustic response of many with lesser brain injuries such as concussion making it more sensitive than CT scan which frequently cannot detect mild injury until the injury progresses. It is this feature of a higher sensitivity to brain injury than CT scan that we have found is most relevant to far forward applications especially in the presence of blast injuries where the brain injury may be ‘mild’ but has the potential to blossom via secondary injury in a significant number of cases.
Implications:
-
The BAM will register ‘positive’ for injuries in persons identified as having TBI by CT scans and neurological exams. It will also indicate some type of disturbance in the acoustic response of many with lesser brain injuries such as concussion making it more sensitive than CT scans.
-
Persons with positive brain acoustic responses and minor injuries will return to ‘normal’ readings over a period of time.
-
The system is useful for both continuous monitoring and triage.
-
Given the simplicity of the system, it is valuable as a portable means for determining effects of concussion and blast as well as time periods over which the effects disappear. As such we anticipate that this will be a very useful tool for use in far forward triage as well as monitoring warfighters to assess the combat readiness status of those who have suffered any type of brain insult from blast, vehicular impact, etc.
- An additional subset of TBI is the identification of persons with elevated ICP using non-invasive techniques. The DoD has recently funded Active Signal in cooperation with our partners, Echodia of France, to develop a combination system using both the BAM and otoacoustic emission technology, specifically cochlear microphonics (CM)11. The combined system will enable the identification of dangerous levels of ICP using the BAM as well as track the change in ICP using CM.
BAM used for Stroke Identification
In addition to the application of acoustic monitoring to TBI, it has also shown promise when applied to stroke. Over the course of a 2 year trial8, all stroke patients admitted to the University of Maryland Medical Center and approximately 60 controls were monitored using the Brain Acoustic Monitor (BAM).
BAM system monitoring a volunteer.
An example of a comparison of a single forehead sensor with a radial sensor output vs. time is shown in Figure 4 below. These traces were obtained from a patient with an ischemic stroke before the administration of rt-PA.
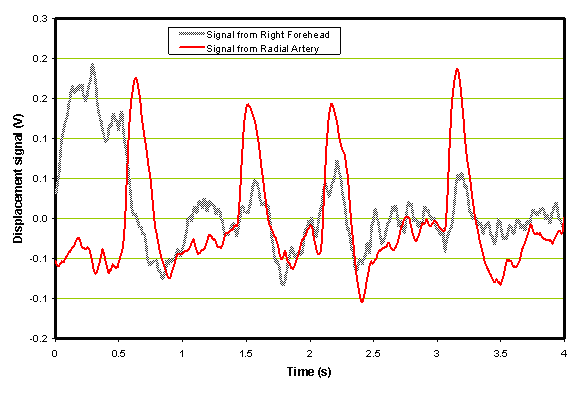
Figure 4. Example of an ischemic patient before the administration of rt-PA.
In the frequency domain shown in Figure 5 the black trace, corresponding to the left side forehead time domain trace, is somewhat higher in the 20 Hz to 60 Hz range than the reference trace from the radial in red.
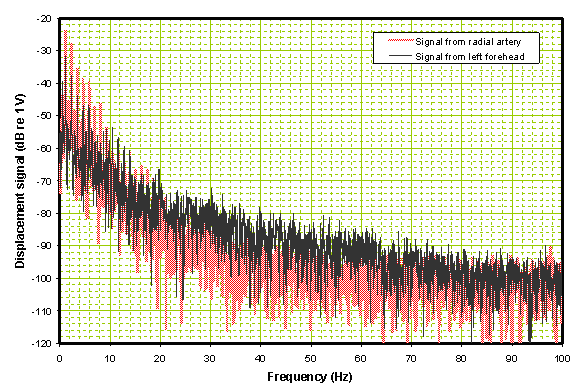
Figure 5. Frequency domain comparison of the two traces in Figure 2.
When the head sensor data is combined with the radial artery data in the frequency domain the result is a divergence from the average response pattern (0 Axis) as shown in Figure 6.

Figure 6: Processed spectrum of Ischemic Patient before administration of rt-PA.
Note that there is a high divergence in the band between 20 and 60 Hz which is common to patients with ischemia. The peak of this divergence is typically > 10 dB. Compare this with the same patient 2 hours later after receiving rt-PA and with improved neurologic status as shown in Figure 7. Note that the time domain data is considerably smoother in the post-rt-PA patient than pre-rt-PA. The FFT is close to a ‘normal’ i.e. well below the 10 dB divergence mark.
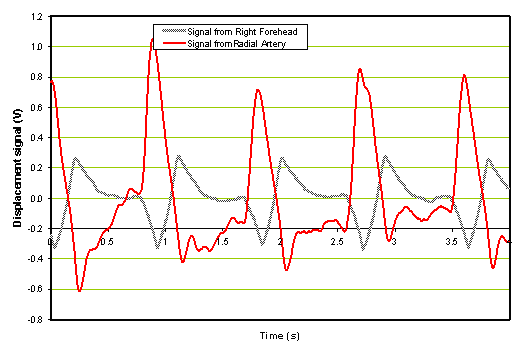
Figure 7a: Time domain signal of ischemic patient 2 hours after administration of rt-PA.
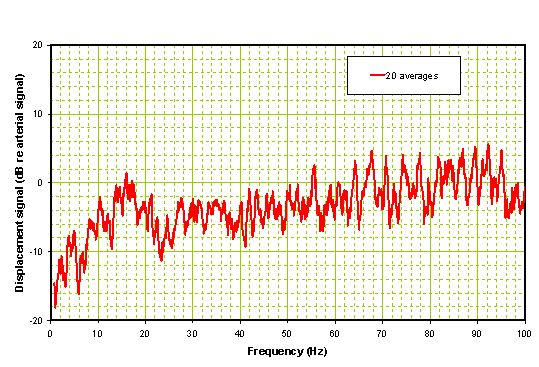
Figure 7b: Processed spectrum of Ischemic Patient 2 hours after administration of rt-PA.
When a typical hemorrhagic patient was monitored, the time domain response and processed frequency domain shown in Figure 8 resulted. In both cases, the signals are close to those of a normal, with some differences in the very low band.
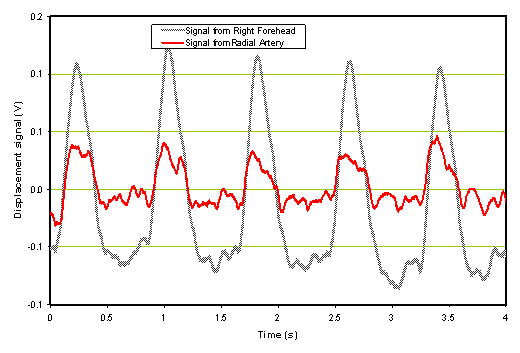
Figure 8a: Time domain signal of hemorrhagic stroke patient
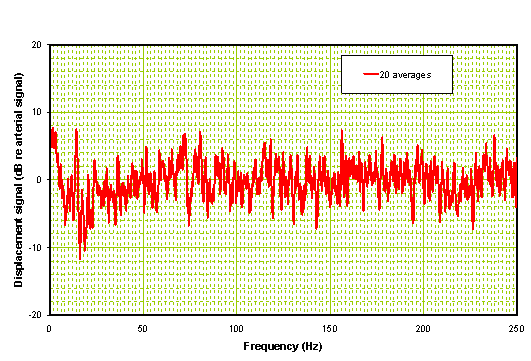
Figure 8b: Processed radial and forehead spectrum of hemorrhagic stroke patient.
The hemorrhagic frequency domain analysis results in a relatively flat response plot with the exception that there is a single spike below 10 Hz. We have observed this < 10 Hz response in one other hemorrhagic and not in ischemics or controls. More important is the complete absence of a significant divergence or hump in the middle of the plot as seen with ischemics.
The results from 100 subjects indicate that there are clear differences in the acoustic response of the brain of ischemic and hemorrhagic patients. It should be noted that the ischemic traits are also present in many TIA patients, consistent with CT and MRI findings. Many of the controls tested positive, but we had no accompanying CT scans that would have enabled us to better understand this result. Accordingly, it will always be necessary to use a combination of a standard stroke scale and the acoustic response in any diagnostic that employs the BAM. When this combination is used, it should generate no false positives among control patients, TIAs, or pseudostrokes. The final item that we are investigating is the small number of hemorrhagic patients that also have acoustic traits proper to ischemic patients. At present we are able to identify other signal properties that distinguish them from ischemic patients, but this effort will not be complete until we have a greater number of hemorrhagic cases within the three hour window after time of stroke onset to establish an adequate database.
The results from this trial of over 100 patients indicate that the combination of a novel acoustic approach and a routine stroke scale may enable stroke subtype identification. Consequently pre-hospital diagnosis of ischemic stroke for the purpose of timely administration of rt-PA could become possible.
In an effort to further the study of this potential application, Active Signal Technologies has worked with the Southeast Coast Ambulance Service9,10 in Sussex, England, to test the usefulness of the BAM in early determination of stroke subtype with the goal of obtaining earlier administration of rt-PA to those with ischemic stroke. This was part of the overall National Health Service initiative to improve stroke care in England, where the same type of initiative with heart disease has shown remarkable results especially in the pre-hospital environment.
Concussion
A particular subset of mTBI is concussion in sports, where the identification of the injury as well as its implications for return to play (RTP) and return to duty are difficult to assess using neuropsychological evaluation alone12. The BAM offers a direct physiologic assessment of any brain disturbance with the potential of placing the assessment of injury on a more direct basis. The Air Force Academy conducted initial studies on some intramural boxers that compared neuropsychological, BAM, and accelerometer testing. On the basis of that experience we have recently begun similar programs with the University of Maryland athletics and are looking toward expanding the application to the high school level.
The software for the BAM system was developed by Active Signal Technologies and Mink Hollow Systems using National Instruments LabVIEW™ for both the laptop and PDA versions of the device. The PDA version of this application was featured in an article written by Mink Hollow Systems and published by National Instruments.
Patents: 5,919,144; 6,491,647; 6,887,199.
References:
1 Maryland Institute for Emergency Medical Services Systems (MIEMSS) 2006-2007 Annual Report.
2 Roudebush, James G. Department of the Air Force Presentation to the U.S. House Armed Services Subcommittee on Military Personnel, February 15, 2008.
3 Roudebush, James G. Department of the Air Force Presentation to the U.S. House Armed Services Subcommittee on Military Personnel, March 14, 2008.
4 Guidelines for the management of severe traumatic brain injury. Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care. J Neurotrauma 17: 451- 627, 2000.
5 Dutton RP, van der Heijden, Aarabi B, Sewell J, Scalea TM, Screening. Screening patients with the Brain Acoustic Monitor: Prediction of CT Scan Findings, Presentation at Trauma East, Ft. Meyers, January 2002.
6 Dutton RP, Sewell J, Aarabi B, Scalea TM, “Preliminary Trial of a Noninvasive Brain Acoustic Monitor in Trauma Patients with Severe Closed Head Injury", Journal of Trauma, November 2002, pp 857-863.
8 Lamonte MP, Sewell J, Bahouth MN, Sewell C, "A Noninvasive Portable Acoustic Diagnostic System to Differentiate Ischemic From Hemorrahagic Stroke", Journal of Neuroimaging, January 2005, Volume 15 Issue 1, pp 57-63
9 South East Coast Ambulance Service (SECAmb) NHS Trust 2006-2007 Annual Report.
10 South East Coast Ambulance Service (SECAmb) NHS Trust 2007-2008 Annual Report.
11 Buki, B, Avan, P. et.al. Non-Invasive Measurements of Intralabyrinthine Pressure changes by electrocochleography and otoacoustic emissions, Hearing Research, 252 2009, pp. 51-59.
12 McCrory, P. et. al. Consensus Statement on Concussion in sport, the 3rd International Conference on Concussion in Sport, Zurich, 2008
